Post Electrophoretic Analysis
Protein Fixation on Gels
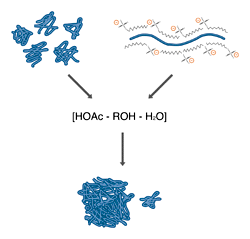
Fixing both native (left) and SDS denatured (right) proteins with acetic acid and alcohol results in an uncoiling of the peptide chains to produce insoluble complexes and monomers.
Fixing (or fixation) is the process whereby proteins are denatured and precipitated in large insoluble aggregates within the gel matrix. Fixation accomplishes several goals. Primarily, fixation prevents the diffusion of proteins, thus keeping the protein bands sharp and resolved during the staining process. In addition, fixation removes gel buffer components, most importantly SDS, which may interfere in the staining process. In some cases, fixatives are used which modify the proteins to enhance the staining reaction.
An ideal fixative is fast, convenient, and nonhazardous to use, and preserves the fine detail of the gel. It is important to be aware that fixing a protein within a gel drastically lowers the amount of protein that can be recovered from that gel after bands have been identified (see guide strip technique). This is probably due to the trapping of gel matrix strands within the denatured protein complexes.
All fixatives operate by causing precipitation of the protein by converting it to an insoluble form. The most commonly used fixatives are solutions of short-chain alcohols and acetic acid in water. The combination of low pH and high organic solvent content disrupts the hydrogen bonding which holds protein structures together and exposes hydrophobic portions of the protein core. The result is an uncoiling of the peptide chain, followed by an essentially irreversible association between chains, producing a high molecular weight complex that is trapped inside the gel. This family of fixatives is cheap and relatively nonhazardous (depending on the alcohol used), and has the additional advantage that many stains are soluble in the fixative. This allows the combination of fixing and staining in one step. The only major drawback is that these solutions are only moderately denaturing, and may not fully fix small or unusually soluble proteins.
Stronger fixatives include trichloroacetic acid (12% in water), sulfosalicylic acid, or formaldehyde. TCA, sulfosalicylic acid and other strong acids act by protonating weak acids in the protein structure, disrupting the salt bridges and charge interactions required to maintain protein secondary structure. Aldehydes, such as formaldehyde and glutaraldehyde, react with amines on the surface of proteins, creating covalent cross-links between protein molecules, resulting in a truly irreversible denaturation
Fixing Proteins on Electrophoresis Gels
Most protein gels can be fixed effectively by soaking for 1 hr in 45% methanol, 45% water, and 10% glacial acetic acid. This solution is stable for up to 30 days at room temperature. A more stable fixative is 25% isopropanol, 65% water and 10% acetic acid, which can be stored at 4°C for up to 4 months.
NOTES: In all cases, agitation during fixing will speed the process by encouraging the penetration of the gel by the fixative. As fixing elutes SDS and other interfering components from the gel, sufficient fixative should be used to dilute these components by at least 5:1. Fixative should be used only once.
Fixing Difficult Proteins
Small or unusually soluble proteins may not be sufficiently fixed by the above protocol. As these proteins diffuse through and out of the gel, smeared bands and loss of sensitivity may result. Prefixing of the gel in 12% trichloroacetic acid for 1-3 hours at room temperature prior to fixing by the above protocol will generally improve the fixing, and hence the staining of such proteins.
In certain cases, where proteins are heavily glycosylated or strongly basic, acid based fixatives may be ineffective. Small peptides may also be resistant even to strong acid fixatives. In such cases an effective alternative to acid precipitation is covalent cross-linking of the proteins with formaldehyde or glutaraldehyde. Formaldehyde fixation may be accomplished in a solution of 25% Ethanol, 15% Formalin (Formalin is 35% formaldehyde), 60% water. Gels are submerged in this solution for 1 hour, and may then be stained with or without subsequent alcohol/acetic acid fixation. Glutaraldehyde is generally used as a fixative in Silver Staining. Gels are soaked in 10% aqueous glutaraldehyde for 30 minutes, then washed twice for 20 minutes with water before staining. This denatures the proteins and fixes them in the gel; it also puts reactive aldehyde groups on the surface of the proteins, which enhance the silver stain reaction.
NEXT TOPIC: Comparing Protein Stains
