The Complete Scintillation Cocktail
Living creatures contain both hydrophobic and hydrophilic compounds, any of which may be labeled during the course of a radioactive experiment. As discussed in earlier sections, the best solvents for scintillation counting are the aromatic organics, such as toluene and xylene. Hydrophobic compounds can be counted directly in such solvents, but hydrophilic materials, which include many biological samples, are completely insoluble in simple cocktails. This requires the engineering of complex cocktails, capable of bringing hydrophilic sample molecules into close proximity to organic solvents and the dissolved scintillators.
Most scintillation cocktails designed for aqueous samples contain surfactants, which emulsify the sample into the organic solvent. Toluene containing the detergent Triton-X100 is a prototypical example of an emulsion cocktail. When water is added to a solution of Triton-X100 in toluene, the detergent molecules orient to form micelles with their hydrophobic alkane chains facing out into the solvent, and their hydrophilic polyethylene glycol chains facing in, "dissolved" in a small amount of trapped water. Various other components are added to the cocktail in small amounts, which regulate the size of the micelles to maintain overall solution clarity.
Due to the surfactants and other additives being generally less effective at energy capture than the solvent, emulsion cocktails are less efficient than pure solvent cocktails. In addition, the partitioning of the aqueous samples into micelles means that the radioactive emissions must escape from the micelle before beginning the scintillation process. Energy is lost while the emitted particle traverses the micelle, resulting in fewer photons per particle reaching the counter. The result is an effective quench, which can be corrected by the means given in the previous section. This quenching is dependent upon the size of the micelles, which in turn depends upon the ratio of sample to cocktail. It is important to use a correction curve which accounts for this volume dependence.
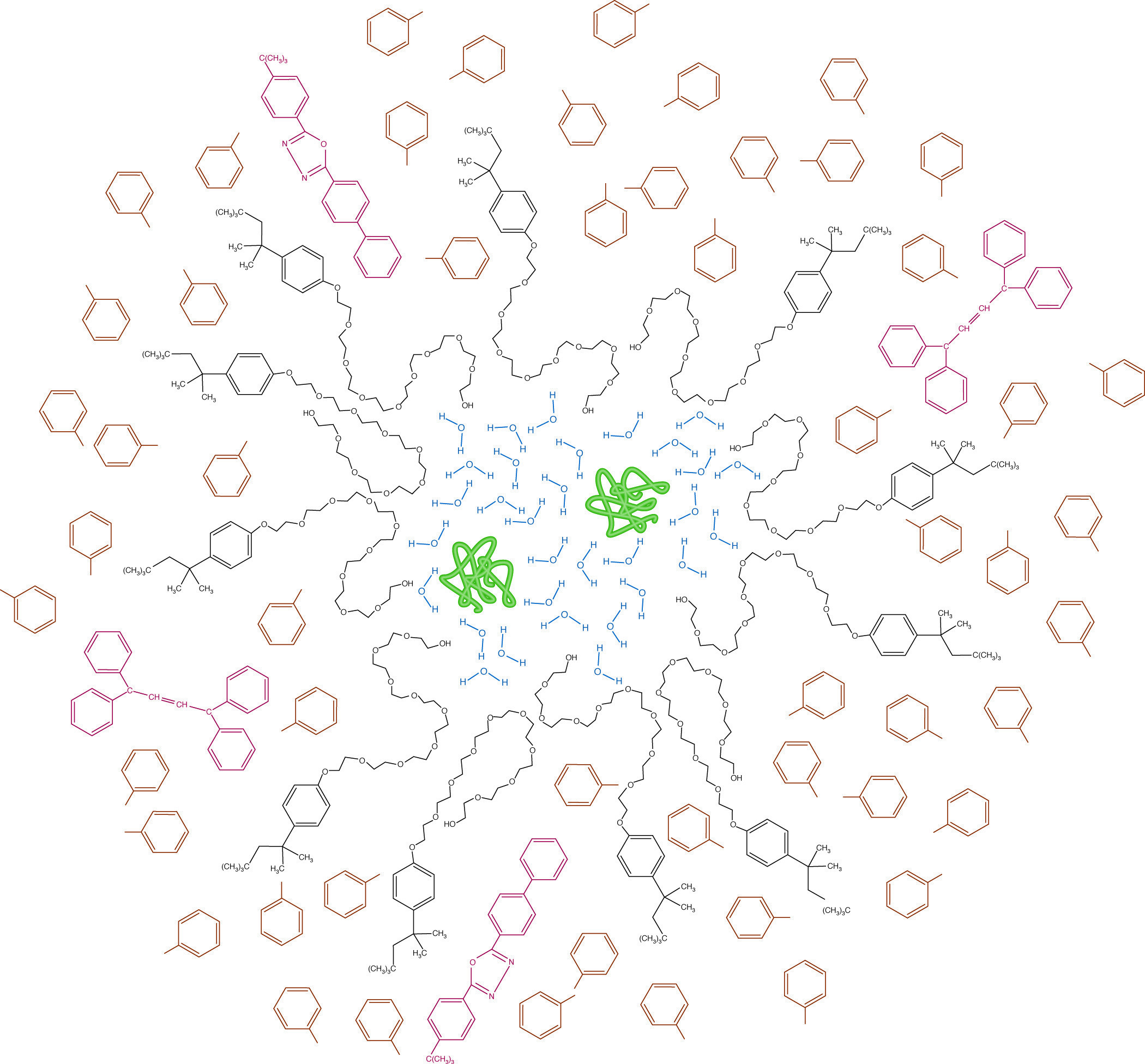
Micellar structure in a scintillation cocktail. Hydrophilic proteins (green) and water (blue) are emulsified by Triton X-100 (black). Radioactive emissions from the labeled protein must pass through the micelle to encounter the toluene solvent (brown) before energy can be passed to the primary and secondary phosphors (red) and be re-emitted as light.
Sample Capacity
Increasing the amount of water dispersed in an emulsion cocktail will increase micellar size, decreasing the energy in any given β particle when it finally escapes the micelle and begins to generate light. At some point, the amount of water added causes a micellar inversion, in which the organic solvent is surrounded by the surfactant, while the water makes up the bulk solution. Efficiency will decrease drastically at this point.
The inversion process generally does not yield a clear solution. Above the cocktail sample holding capacity, the mixture is cloudy or opaque, and photons emitted within such a solution are lost to internal reflectance. Sample holding capacity is dependant upon sample composition and upon temperature. In planning scintillation counting experiments, it is crucial to ensure that the sample volume not be too close to the capacity of the cocktail. Such samples may turn opaque with a 1-2° change in temperature, and give falsely low readings. With the use of translucent plastic scintillation vials this type of artifact can be very difficult to detect.
| Sample | Capacity (ml sample/10 mL cocktail) |
|---|---|
| Water (20 oC) | 4.5 |
| Water (25 oC) | 4.0 |
| Water (15 oC) | 5.0 |
| 0.05M Tris-HCl | 4.5 |
| 0.15M NaCl | 4.0 |
| 10% Sucrose | 3.0 |
| 8M Urea | 1.0 |
NEXT TOPIC: Chemiluminescence and Static Electricity
