Electrophoresis
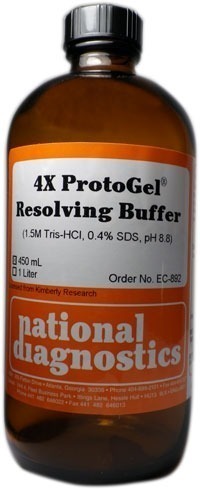
ProtoGel Resolving Buffer (4X)
$48.00 – $84.00
Catalog number: EC-892
- Traditional Laemmli Resolving Gel Buffer (4X)
- Formulated with 0.2 Micron Filtration
- Ultra-Pure Reagents in 18 MegOhm Water
- Crystal Clear, Reproducible Gels
Description
Catalog number: EC-892
- Traditional Laemmli Resolving Gel Buffer (4X)
- Formulated with 0.2 Micron Filtration
- Ultra-Pure Reagents in 18 MegOhm Water
- Crystal Clear, Reproducible Gels
The use of National Diagnostics’ ProtoGel Resolving Buffer will ensure the purity and performance of your Laemmli gels. When diluted as directed ProtoGel Resolving Buffer forms a gel of 0.375 M Tris-HCl and 0.1% SDS, pH 8.8.
Storage: ProtoGel Resolving Buffer is stable for 24 months when stored tightly capped in a dark area at room temperature.
Additional information
| Weight | 2.8 lbs |
|---|---|
| Dimensions | 7 × 7 × 14 in |
Protocol
The volume of ProtoGel required for gel casting solutions of any volume and acrylamide concentration may be calculated from the following formula:
Vp =(X) (Vt)/40
where:
Vp = Volume of 40% ProtoGel
X = % Monomer Desired in Gel
Vt = Total Volume of Gel Casting Solution
EXAMPLE: To make 100 ml of a 10% monomer gel, calculate the volume of ProtoGel to add as follows:
Vp = (10) (100)/40 = 25.0 ml
Initiate and Cast Gel:
For optimal results degas gel solution for 10 minutes under vacuum aspiration prior to innitiation with APS and TEMED. Add 1.0ml of 10% (w/v) ammonium persulfate for every 100ml of gel casting solution. Swirl gently to mix. Add 0.1 ml of TEMED for every 100ml of gel casting solution. Swirl gently to mix. Pour the solution into the gel casting cassette. The gel should begin to set in 10-20 minutes. To provide a sharp interface, overlay the gel with water saturated n-butanol during polymerization. Flush butanol away with water just before casting the stacking gel (below).
Cast Stacking Gel:
Use ProtoGel Stacking Buffer to make 10ml of a 4% stacking gel:
- ProtoGel: 1.00ml
- ProtoGel Stacking Buffer: 2.50ml
- Deionized water: 6.44ml
Add 0.05ml 10% ammonium persulfate and 0.01ml of TEMED. Gel will begin to set in 20 minutes.
Safety Overview
Safety Summary (see SDS for complete information before using product):
EMERGENCY OVERVIEW – IMMEDIATE HAZARD
WARNING! HARMFUL IF SWALLOWED. ACRYLAMIDE MAY CAUSE NERVOUS SYSTEM DAMAGE. MAY CAUSE ALLERGIC SKIN REACTION. MAY CAUSE EYE IRRITATION. POLYMERIZATION MAY OCCUR FROM EXCESSIVE HEAT OR CONTAMINATION.
- UV Shadowing
- Using PAGE to Determine Nucleic Acid Molecular Weight
- Uneven Staining
- The Polyacrylamide Matrix-Buffer Strength
- The Polyacrylamide Matrix
- The Mechanical and Electrical Dynamics of Gel Electrophoresis — Electrophoresis System Dynamics
- The Mechanical and Electrical Dynamics of Gel Electrophoresis – Ohm’s Law
- The Mechanical and Electrical Dynamics of Gel Electrophoresis – Intro and Sample Mobility
- The Electrophoresis Matrix
- The Agarose Matrix
- Staining Proteins Immobilized on Membranes
- Staining Protein Gels with Coomassie Blue
- SSCP Analysis
- Southern Blotting
- Smeared Bands
- Silver Staining Protein Gels
- Silver Staining DNA Gels
- Sanger Sequencing
- Sample Preparation for SDS-PAGE
- Sample Preparation for Native Protein Electrophoresis
- Sample Preparation for Native PAGE of DNA
- Sample Prep for Denaturing PAGE of DNA
- S1 Mapping
- Run Conditions in Denaturing PAGE
- RNA Mapping
- RNA Electrophoresis
- Ribonuclease Protection
- Restriction Digest Mapping
- Radioactive Emissions and the Use of Isotopes in Research
- Protein Fixation on Gels
- Primer Extension
- Preparing Denaturing DNA & RNA Gels
- Preparation of Denaturing Agarose Gels
- Preparation of Agarose Gels
- Pouring Sequencing Gels
- Post-Electrophoretic Visualization with Nuclistain
- PFGE and FIGE
- Peptide Mapping
- PCR Analysis: Yield and Kinetics
- PCR Analysis: An Examination
- Overview of Western Blotting
- Northern Blotting
- Native Protein Electrophoresis
- Native PAGE of DNA
- Multiphasic Buffer Systems
- Mobility Shift Assay
- Methylation & Uracil Interference Assays
- Method for Western Blotting
- Mechanism of Immunostaining
- Mechanism of Immunostaining
- Measuring Molecular Weight with SDS-PAGE
- Maxam & Gilbert Sequencing
- Manual Sequencing
- Isotachophoresis
- Isoelectric Focusing
- In Gel Enzyme Reactions
- Immunostaining with Alkaline Phosphatase
- Immuno-Electrophoresis / Immuno-Diffusion
- Horizontal and Vertical Gel Systems – Vertical Tube Gels
- Horizontal and Vertical Gel Systems – The Vertical Slab Gel System
- Horizontal and Vertical Gel Systems – The Horizontal Gel System
- Homogeneous Buffer Systems
- Heteroduplex Analysis
- Guide Strip Technique
- Gel Preparation for Native Protein Electrophoresis
- Gel Preparation for Native PAGE of DNA
- Gel Electrophoresis of RNA & Post Electrophoretic Analysis
- Gel Electrophoresis of PCR Products
- Faint bands, low background
- Faint Bands, High Background
- Ethidium Bromide Staining
- Enzyme Linked Immunosorbent Assay (ELISA)
- Electrophoresis Buffers-Choosing the Right Buffer
- Electrophoresis Buffers–The Henderson-Hasselbalch Equation
- DNase I Footprinting
- DNA/RNA Purification from PAGE Gels
- DNA/RNA Purification from Agarose Gels – Electroelution
- Differential Display
- Denaturing Protein Electrophoresis: SDS-PAGE
- Denaturing Polyacrylamide Gel Electrophoresis of DNA & RNA
- Coomassie Blue Stain- Troubleshooting
- Conformational Analysis
- Casting Gradient Gels
- Buffer Additives-Surfactants
- Buffer Additives-Reducing Agents
- Buffer Additives-Hydrogen Bonding Agents
- Blotches on Gel
- Biological Macromolecules: Nucleic Acids
- Biological Macromolecules – Proteins
- Autoradiography
- Autoradiographic Enhancement with Autofluor
- Automated Sequencers
- Analysis of DNA/Protein Interactions
- An Overview of Northern and Southern Blotting
- Alkaline Blotting
- Agarose Gel Electrophoresis of DNA and RNA – Uses and Variations
- Agarose Gel Electrophoresis of DNA and RNA – An Introduction
- Activity Stains