Post Electrophoretic Analysis
Overview of Western Blotting
Proteins can also be detected immunologically following electrophoresis, a technique known as Western blotting. This method relies on the fact that most epitopes (sites recognized by antibodies, generally comprising several amino acids) are still recognizable following denaturing of the protein with SDS and binding to the surface of a membrane.
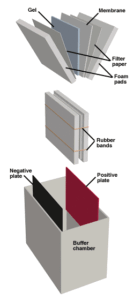
The Western Blotting apparatus. Proteins are electrophoresed from the gel to the membrane.
The two primary advantages of Western blotting are sensitivity and specificity. Silver staining may detect 10ng of protein, and it will detect all proteins in a given sample. Western blotting can detect as little as 0.1ng of protein, and it will selectively detect only the protein of interest. Thus a complex mixture containing only traces of the desired protein may be analyzed accurately with this technique.
Antibodies are large molecules and penetrate gels slowly. In order to efficiently use immunological staining for post-electrophoretic detection, the proteins in the gel must be immobilized on an exposed surface, a process called "blotting." Electroblotting is commonly used in the western system. Following the run, the gel is sandwiched with a membrane, and a voltage is applied perpendicular to the plane of the gel, such that proteins are electrophoresed out of the gel and onto the membrane.
Various membranes are employed in Western blotting, but the two predominant types are nitrocellulose and PVDF (poly vinylidene difluoride). Nitrocellulose was the first support used for Western blotting and is still widely used. It probably binds proteins through hydrophobic interactions, the binding is blocked by oils. Nitrocellulose has a relatively high capacity for protein ( 50 - 100 µg/cm2 ), but it is also brittle and difficult to work with. In addition, the binding of proteins to the membrane has proven reversible under some circumstances, leading to sample loss and the consequential lowering of detection limits.
PVDF is mechanically stronger than nitrocellulose. It binds proteins more tightly and has up to two-fold higher capacity. Proteins immobilized on PVDF can be analyzed by microsequencing or amino acid analysis in addition to western blotting. The PVC-like nature of this material gives it a resistance to mechanical and chemical stresses and provides hydrophobic "pockets" for denatured protein binding. For these reasons, PVDF has gained considerable popularity for Western blotting use.
Once the protein has been transferred to the membrane, the membrane (or blot) is blocked to prevent unoccupied protein binding sites from nonspecifically immobilizing antibodies in the following steps. Blocking generally is carried out by incubating the blot in skim milk, BSA, or some other complex mixture of proteins and surfactants such as National Diagnostics' ProtoBlock System. The blocked blot is then ready for antibody probing.
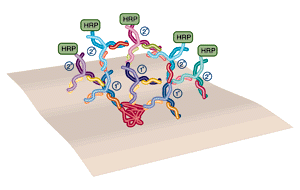
In western blotting, the use of polyclonal primary and secondary antibodies results in amplification of the signal.
In order to achieve maximum sensitivity most western blots are probed in 2 steps, using a "primary" and "secondary" antibody (see figure below). The primary antibody detects the protein of interest. If this antibody is "polyclonal" (that is, derived from an immunized animal, and therefore containing antibodies to multiple epitopes on the same protein) many antibodies will bind to each protein molecule. The secondary antibody is directed against the primary antibody and is generally polyclonal, so several 2° antibodies will bind each 1° antibody. The result is an amplification, in which many 2° antibodies are bound to the site of each protein. The 2° antibody carries the enzyme label.
Following binding and washing, detection reagents are applied to the gel to visualize the band(s) detected. Chromogenic substrates cause colored bands to appear on the white blot. If luminescent systems are used, the blot is placed against a piece of X-ray film and exposed, typically for 1 - 10 minutes.
NEXT TOPIC: Method for Western Blotting
- UV Shadowing
- Uneven Staining
- Staining Proteins Immobilized on Membranes
- Staining Protein Gels with Coomassie Blue
- Southern Blotting
- Smeared Bands
- Silver Staining Protein Gels
- Silver Staining DNA Gels
- Protein Fixation on Gels
- Post-Electrophoretic Visualization with Nuclistain
- Overview of Western Blotting
- Northern Blotting
- Method for Western Blotting
- Mechanism of Immunostaining
- Mechanism of Immunostaining
- Immunostaining with Alkaline Phosphatase
- Guide Strip Technique
- Faint bands, low background
- Faint Bands, High Background
- Ethidium Bromide Staining
- Enzyme Linked Immunosorbent Assay (ELISA)
- Coomassie Blue Stain- Troubleshooting
- Blotches on Gel
- Autoradiography
- Autoradiographic Enhancement with Autofluor
- An Overview of Northern and Southern Blotting
- Alkaline Blotting
