Fundamentals of Liquid Scintillation Counting
Measurement of Radiation and Isotope Quantitation
Most research applications of radioisotopes require an eventual quantitation of the isotope, which is done by measuring the intensity of radiation emitted. Common nomenclature expresses this intensity as disintegrations per minute (DPM). The becquerel (Bq) is the SI unit for radiation, corresponding to 60 DPM (one disintegration per second). The curie, an earlier and still prevalent measure, is equal to 3.7 x 1010 Bq.
Truly accurate measurement of DPM would require that every emission event be detected and counted, which is not possible in most situations. Additionally, naturally occurring isotopes and cosmic radiation contribute significant "background" radiation. Corrections for efficiency and background are needed to convert the counts per minute measured (CPM) into DPM. Techniques have been developed for applying these corrections, and a great deal of research has been carried out to improve the efficiency of counting, using various detection systems.
Ionization Detection
Alpha, beta and gamma radiation all fall into the category of ionizing radiation. Alpha and beta particles directly ionize the atoms with which they interact, adding or removing electrons. Gamma-rays cause secondary electron emissions, which then ionize other atoms. The ionized particles left in the wake of a ray or particle can be detected as increasing conductivity in an otherwise insulating gas, which is done in electroscopes, ionization chambers or proportional counting chambers. These devices measure the pulse of conductivity between two electrodes when a particle or ray ionizes the gas between them. If a sufficiently high voltage is applied between the electrodes, an amplification of the signal can be obtained, and such counters can be quite sensitive. Their utility is severely limited by the fact that for most research applications only gas phase isotopes can be detected. This greatly complicates sample preparation (requiring the combustion of 14C to 14CO2, for example) and may preclude the analysis of some compounds entirely.
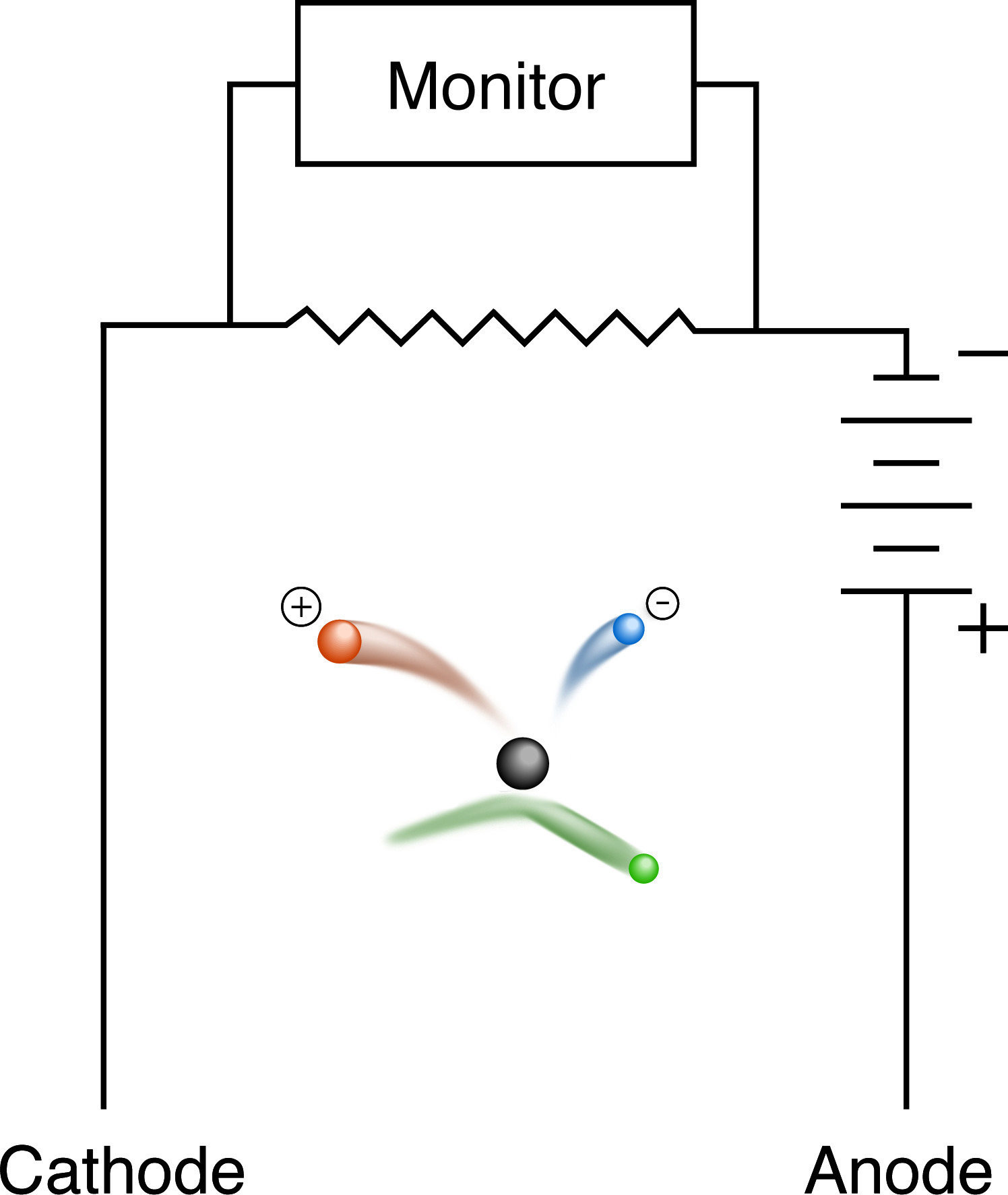
Scintillation Detection
Some irradiated atoms are not fully ionized by collision with emitted particles, but instead have electrons promoted to an excited state. (A sub population of ionized atoms can recombine with an ion of opposite sign and also produce an excited state.) Excited atoms can return to ground state by releasing energy, in some cases as a photon of light. Such scintillation phenomena form the basis of a set of very sensitive radiation detection systems.
In solid scintillation systems, a crystal of inorganic or organic material—the scintillator—is irradiated by the sample. The light emitted in response to this irradiation is taken as a measure of the amount of radioactivity in the sample.
Solid scintillation is excellent for γ radiation which is highly penetrating and can cause scintillation throughout a large crystal. An advantage of these techniques is that the same crystal is used for each sample, which enhances reproducibility. Unlike ionization counting, a gas phase sample is not required.
For α or β counting, however, solid scintillation has severe limitations. The crystal must be protected from contamination by the sample, which means that the α & β particles must traverse a barrier prior to reaching the scintillator. α-rays in particular are severely attenuated by even 0.05mm of aluminum or copper, and so cannot be expected to reach a scintillator crystal through even the thinnest shielding.
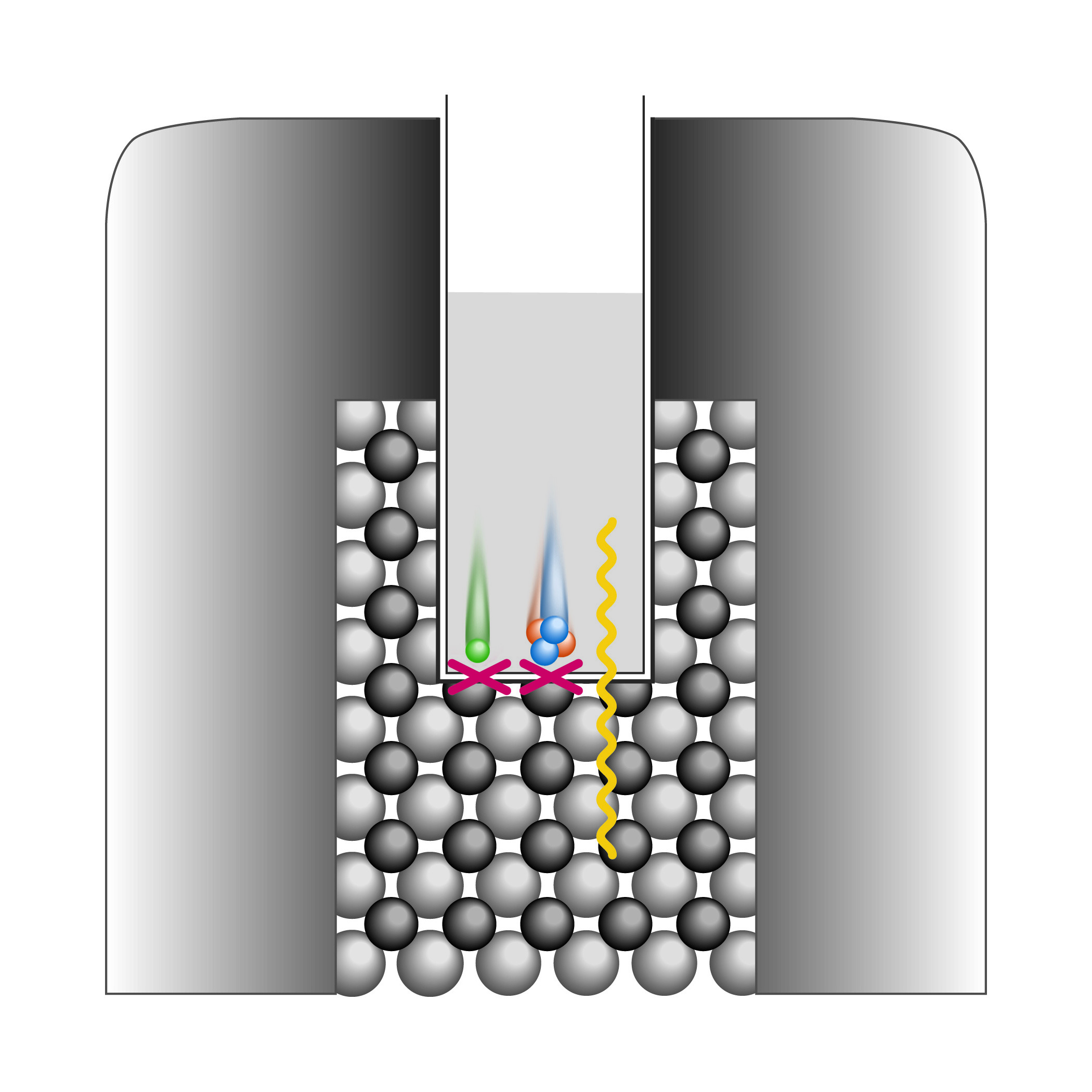
Liquid scintillation (LSC), detailed in the next section, was evolved to provide a usable method for counting organic isotopic compounds. These materials are most often water soluble b-emitters. LSC addresses the need for convenience, reproducibility, and high sensitivity in these assays. It also offers solutions to the problem of counting aqueous (i.e. biological) samples in a nonaqueous environment.
NEXT TOPIC: Mechanism of Liquid Scintillation Counting
