Applications of Liquid Scintillation Counting
Liquid Scintillation and Radiation Safety
Working with radioactive isotopes requires diligent attention to safety measures, in order to avoid hazardous exposure(s). Because radioactivity cannot be detected without instrumentation, spills can easily be spread through and even out of the lab before they are noticed. Safety in radioisotope work requires sufficient attention to both containment and surveillance. Containment measures are designed to prevent the release of isotopes in unprotected areas. Surveillance aims to detect such accidental releases as rapidly as possible to prevent the spread of contamination.
Containment and Monitoring
Containment measures are taught in radiation safety courses. A brief summary is presented here, which is not intended as a substitute for such a course. The primary safeguard against radioactive spills is common sense. Radioactivity should be handled only in designated areas, which should be covered with disposable absorbent pads. Droplets of radioactive solutions will be absorbed and trapped by the pads, which are then disposed of as solid radioactive waste. Users of radioactivity must wear lab coats, gloves and eye protection. Gloves should be monitored with a Geiger counter if the isotope emissions are detectable by this means. In any case, frequent changing of gloves if contamination is detected or suspected will keep exposure to a minimum. Shielding should be used for 125I and 32P work, or for any isotope whose emissions can penetrate skin, but it is important to keep in mind that the primary long term danger from radioactive components is through ingestion or skin absorption. Eating or drinking or even chewing gum must be excluded from radioactive use areas.
Surveillance begins with personal dosimeters and Geiger counters. Dosimeters are most often film badges and film rings. These are worn during radioactive experiments, and the film contained within them is exposed by the emissions which affect the worker. A correlation between film exposure and worker exposure allows the detection of dangerous levels of radiation in the lab. Radiation safety departments also use film badges to ensure that no user exceeds their long term exposure limits in a given year. Short term monitoring of exposure to high-energy emissions can be done with a Geiger counter, which detects the ionization of a gas in a sealed tube. Only those emissions which are energetic enough to penetrate the tube can be detected: 32P and the highest energy emissions from 35S. Geiger counters can be invaluable for checking gloves for contamination during the course of an experiment.
Wipe Testing
Once an experiment is finished, a comprehensive and sensitive check of all work areas is required. This is accomplished by use of "wipe tests". A 4 cm2 piece of paper or other absorbent material is rubbed vigorously over the work area, placed in scintillation cocktail and counted. If counts above background are detected, the contaminated area is subdivided and the divisions wipe tested. Contaminated areas are cleaned and retested until no contamination can be detected.
The type of filter used in wipe testing has a marked effect on the reliability of the results obtained. Often standard filter paper discs are used. Such discs generally adhere to the side or the bottom of the scintillation vial. If the vial is placed in the counter such that the filter is on the side facing the photomultiplier tube, much of the light emitted by the cocktail will be absorbed by the filter. This will give artificially low numbers of counts, measuring contaminated areas as clean. This hazard is avoided by the use of a wipe which dissolves in the scintillation cocktail such as National Diagnostics' Nuc-Wipes.
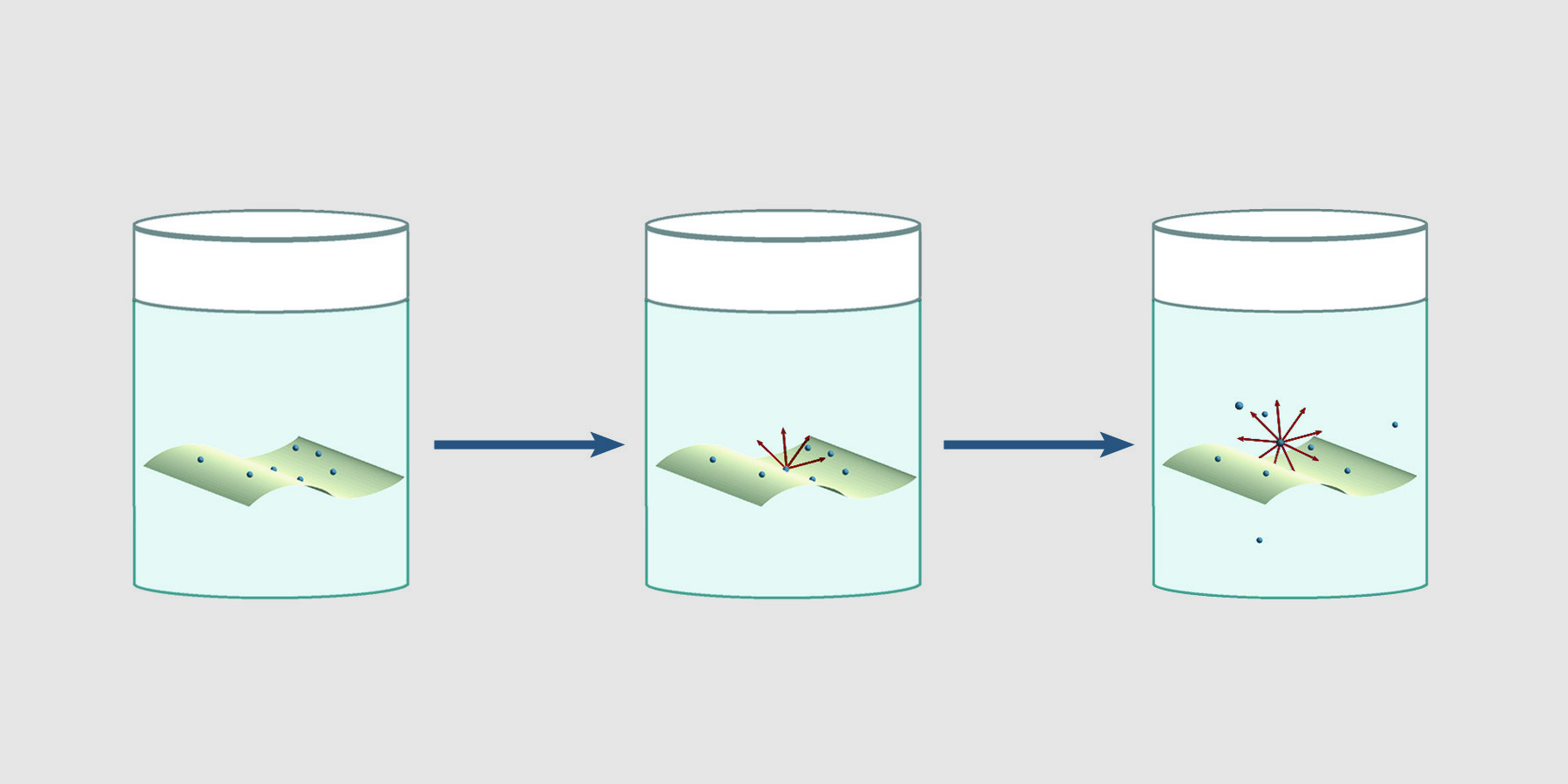
Using intact filters for environmental wipe tests can give inaccurate, erratic results. β particles originating from particles on intact filters are attenuated and absorbed by the filter. Furthermore, depending on the relative affinity of the material for the solution, as material leaves the filter for the solution, counts change over time.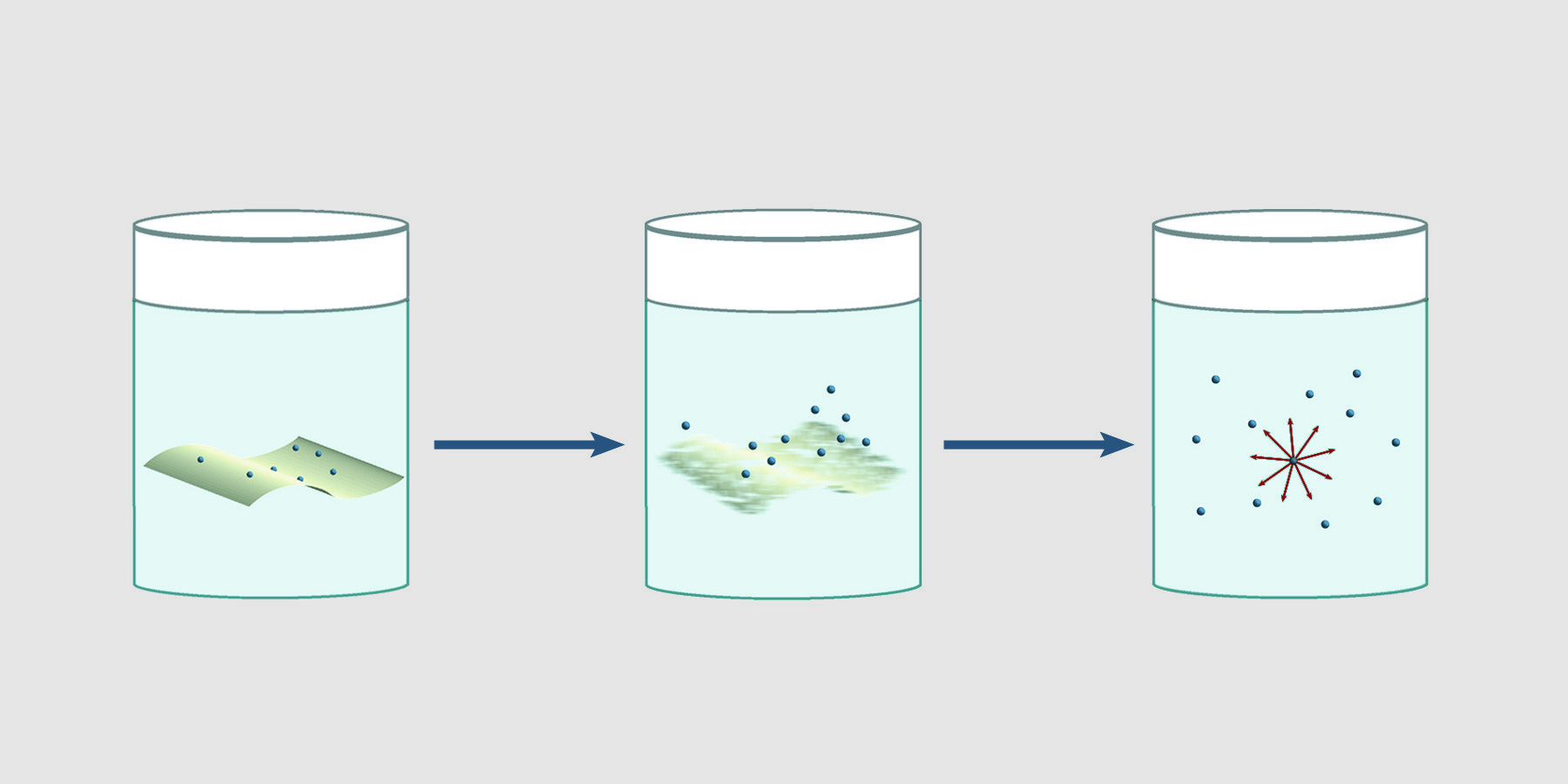 Nuc-Wipes eliminate the dependence of results on the direction of the filter paper and time. Since Nuc-Wipes dissolve in scintillation fluid, there is no intact filter to absorb or attenuate beta emissions. 4π counting efficiency is achieved, giving reproducible results.
Nuc-Wipes eliminate the dependence of results on the direction of the filter paper and time. Since Nuc-Wipes dissolve in scintillation fluid, there is no intact filter to absorb or attenuate beta emissions. 4π counting efficiency is achieved, giving reproducible results.
Efficient and effective cleaning of spills requires some knowledge of the chemical nature of the labeled compound. Water soluble materials will come off of nonabsorbent surfaces with detergent solutions. Hydrophobic compounds require the use of higher detergent concentrations. Extremely hydrophobic compounds or absorbent surfaces may require the use of organic solvents. If an unknown sample is spilled (spent culture medium, cellular extracts, etc.), a general purpose radioactive decontamination agent should be tried, such as National Diagnostics' Nuclean, followed by solvents if necessary.
- Waste Disposal Issues in Scintillation Counting
- Preparing Tissue Samples for Scintillation Counting
- Preparing Samples in PAGE Gels for LSC
- Liquid Scintillation and Radiation Safety
- HPLC Flow Counting
- Counting Samples on Cellulose-Ester Filters
- Counting Samples from TLC Plates by LSC
- Counting Carbon Dioxide by LSC
- Assaying Discrete Samples by Liquid Scintillation Counting
