Fundamental Principles of Electrophoresis
Buffer Additives-Hydrogen Bonding Agents
In most forms of electrophoresis, the solution perfusing the gel matrix typically contains one or more substances in addition to the buffer salts. Serving the purpose of modifying the properties of sample molecules, these additives can be categorized as hydrogen bonding agents, surfactants, or reducing agents. Hydrogen bonding agents Urea or formamide can be introduced…
Read MoreMultiphasic Buffer Systems
At the start of multiphasic SDS-PAGE protein electrophoresis, the anions are chloride (green) in the stacking and resolving gels and glycine (orange) in the tanks. Employing gel and buffer discontinuities to produce sharp separation among sample components, multiphasic electrophoresis design can improve the resolution of electrophoresis (especially protein electrophoresis). The system employs a separating gel…
Read MoreHomogeneous Buffer Systems
In a homogeneous buffer system, the identity and concentration of buffer components are the same in the gel and the tanks. Most forms of DNA and RNA electrophoresis generally use homogeneous buffer systems. Electrophoresis of proteins is most often performed under multiphasic conditions, where tank and gel buffers differ. In a homogeneous system, the buffer…
Read MoreElectrophoresis Buffers-Choosing the Right Buffer
Several factors to consider when choosing a buffer include: 1) pKa value – A buffer should be chosen with a pKa that is very close to the desired pH, preferably within a half point. The buffer will have the greatest capacity both to absorb or release protons with the acid and the base form well…
Read MoreThe Polyacrylamide Matrix-Buffer Strength
The buffer system in electrophoresis controls the pH of the gel, preventing damage to sample molecules and, in certain cases, controlling the ionization state of the molecules. A second, though no less significant function, derives from the fact that the vast majority of current flowing through the electrophoresis gel is carried by the buffer ions.…
Read MoreElectrophoresis Buffers–The Henderson-Hasselbalch Equation
In its simplest form, a buffered solution contains a mixture of a weak acid and its conjugate base. The position of acid/base equilibrium is represented by the acid dissociation constant, Ka. This number is large if the acid is stronger and equilibrium tends toward dissociation. It is small for an equilibrium that tends toward proton…
Read MoreThe Agarose Matrix
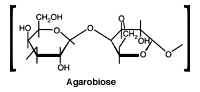
Agarobiose is the basic unit of the agarose. A natural colloid extracted from seaweed, agarose is a linear polysaccharide made up of the repeating unit agarobiose, which consists of alternating units of 1,3-linked b-D-galactopyranose and 1,4-linked 3,6-anhydro-a-L-galactopyranose. Gels prepared from agarose have a substantially larger pore size than polyacrylamide gels. Agarose gels can be prepared…
Read MoreThe Polyacrylamide Matrix
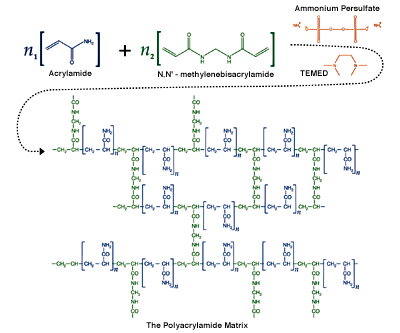
Polyacrylamide gels are formed by the polymerization of acrylamide in an aqueous solution in the presence of small amounts of a bifunctional crosslinker. The crosslinker is usually methylenebisacrylamide (bis, or MBA). The polymerization of a polyacrylamide matrix with methylenebisacrylamide cross-linking. Polyacrylamide gels are formed by the polymerization of acrylamide in aqueous solution in the presence…
Read MoreThe Electrophoresis Matrix
In-gel electrophoresis the matrix forces sample components to separate by size, as they move through its porous structure. The matrix provides greater resistance to the movement of larger molecules. It also performs additional functions including the reduction of convection currents and the inhibition of sample diffusion, encouraging the separated components to remain as sharp, distinct…
Read MoreThe Mechanical and Electrical Dynamics of Gel Electrophoresis – Ohm’s Law
Ohm’s Law: Relationships between electrical parameters Ohm’s law describes the relationship between the voltage, V, the current, I, and the resistance, R, in a DC circuit. A greater voltage produces a greater proportional current through a given resistor: V = IR A variation of Ohm’s law describes how small changes in electrical current can produce…
Read More