Post Electrophoretic Analysis Articles
Buffer Additives-Hydrogen Bonding Agents
In most forms of electrophoresis, the solution perfusing the gel matrix typically contains one or more substances in addition to the buffer salts. Serving the purpose of modifying the properties of sample molecules, these additives can be categorized as hydrogen bonding agents, surfactants, or reducing agents.
Hydrogen bonding agents
Urea or formamide can be introduced to electrophoresis samples prior to loading or to the gel buffer itself in order to cleave hydrogen bonds. These substances disrupt hydrogen bonds by occupying the bonding sites themselves. Hydrogen bonds are dipole-dipole attractions that occur between polar, hydrogen-containing functional groups such as amine or hydroxyl groups. Hydrogen bonding has a major influence on the conformation and solubility of biological molecules. It is frequently necessary to include one or both of these substances to standardize sample conformation or to solubilize samples.
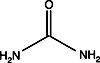
Urea
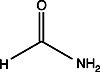
Formamide
In denaturing DNA and RNA electrophoresis, formamide plus heat in the sample preparation stage followed by urea in the gel buffer are employed to disrupt the hydrogen bonding relationships central to base pairing. By substituting their own hydrogen bonding relationships with sample molecule functional groups, formamide and urea cause the separation of the complementary strands in double-stranded DNA and RNA and, furthermore, disrupt the kinks and loops in single-stranded species brought about by self-annealing. The resulting molecules are long and straight, and the influence of small differences in conformation on electrophoretic mobility is minimized. Because of the importance of this technique in electrophoresis, the terms "urea gel" and "denaturing gel" are often used interchangeably in the laboratory.
Urea can be employed in protein gels if the sample molecules are insoluble or aggregated, although detergents can also be used. A downside to the use of urea with proteins can be the formation of cyanate ions which will react with some proteins, although Tris buffers will effectively protect the protein samples. If Tris buffers cannot be used, pre-running the gel for 30-40 minutes before adding samples or treating the solution with ion exchange resin before mixing the gel solution can also effectively solve this problem.
NEXT TOPIC: Buffer Additives: Surfactants
- The Polyacrylamide Matrix-Buffer Strength
- The Polyacrylamide Matrix
- The Mechanical and Electrical Dynamics of Gel Electrophoresis — Electrophoresis System Dynamics
- The Mechanical and Electrical Dynamics of Gel Electrophoresis – Ohm’s Law
- The Mechanical and Electrical Dynamics of Gel Electrophoresis – Intro and Sample Mobility
- The Electrophoresis Matrix
- The Agarose Matrix
- Radioactive Emissions and the Use of Isotopes in Research
- Multiphasic Buffer Systems
- Horizontal and Vertical Gel Systems – Vertical Tube Gels
- Horizontal and Vertical Gel Systems – The Vertical Slab Gel System
- Horizontal and Vertical Gel Systems – The Horizontal Gel System
- Homogeneous Buffer Systems
- Faint bands, low background
- Faint Bands, High Background
- Ethidium Bromide Staining
- Electrophoresis Buffers-Choosing the Right Buffer
- Electrophoresis Buffers–The Henderson-Hasselbalch Equation
- Coomassie Blue Stain- Troubleshooting
- Buffer Additives-Surfactants
- Buffer Additives-Reducing Agents
- Buffer Additives-Hydrogen Bonding Agents
- Biological Macromolecules: Nucleic Acids
- Biological Macromolecules – Proteins
