Post Electrophoretic Analysis Articles
Buffer Additives-Surfactants
A crucial initial step in the electrophoretic separation of proteins is the solubilization of the sample molecules. This is especially true if there are extensive nonpolar interactions. Although urea in high concentration was often employed in the past for this purpose, researchers now often have recourse to the use of nonionic, anionic, or cationic detergents.
Nonionic detergents, such as Tween-20 or Triton X-100, are generally less strongly denaturing than anionic or cationic detergents. Researchers use nonionic surfactants to preserve enzyme activity or some delicate immunological properties that anionic or cationic detergents would destroy. Generally, Tween-20 or Triton X-100 are added sparingly to the gel buffer (0.1%). These substances can also be employed in a 1% solution for the pretreatment of samples.
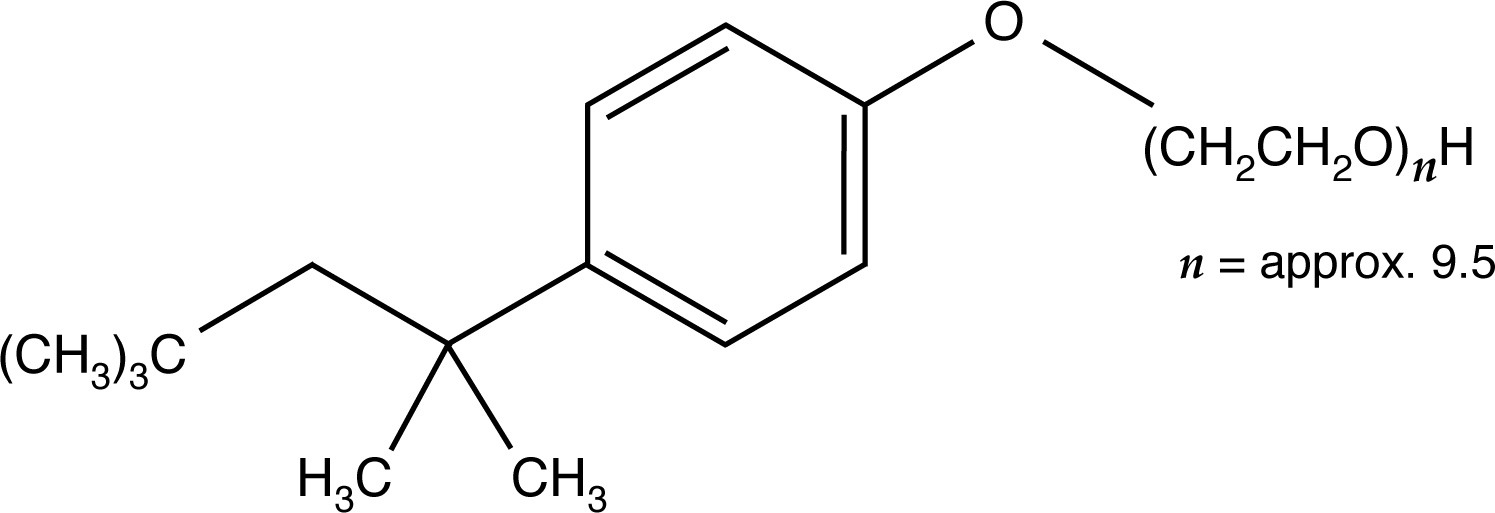
A major drawback with nonionic detergents is that, unlike charged surfactants, these detergents produce no consistent charge to mass ratio among sample molecules for electrophoresis. For this reason, the molecular weight of proteins cannot be directly determined by one electrophoretic run, and, in general, electrophoresis results are more difficult to interpret than results from electrophoresis that has been carried out in the presence of charged surfactants such as SDS (sodium dodecyl sulfate).
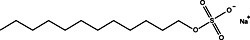
By far the most commonly employed detergent additive in protein electrophoresis is the anionic surfactant SDS (sodium dodecyl sulfate). Proteins under treatment with SDS become completely blanketed by negatively charged dodecyl sulfate anions, unwinding to assume an extended conformation. The number of bound detergent molecules is quite large, approaching half the number of amino acid residues. As a result, the intrinsic charge of treated proteins becomes overwhelmed by the charge of the surfactant molecules, and even proteins of the widely divergent structure have a virtually uniform charge to mass ratio. Electrophoresis of such samples results in strict separation by molecular weight.

Although rarely necessary, cationic surfactants such as CTAB, and cetyltrimethylammonium bromide, can be used for the electrophoresis of samples posing difficulties for SDS-PAGE. Such cases include the electrophoresis of either extremely acidic or extremely basic samples. Being very negatively charged, extremely acidic samples can exhibit poor binding with SDS. The problem with extremely basic samples is that the addition of SDS can lead to precipitation. The use of CTAB as an alternative carries the same benefit of SDS in that a uniform charge to mass ratio among sample molecules is produced, although the apparatus will need to be adjusted to allow samples to migrate toward the negative pole rather than the positive pole.
NEXT TOPIC: Buffer Additives: Reducing Agents
- The Polyacrylamide Matrix-Buffer Strength
- The Polyacrylamide Matrix
- The Mechanical and Electrical Dynamics of Gel Electrophoresis — Electrophoresis System Dynamics
- The Mechanical and Electrical Dynamics of Gel Electrophoresis – Ohm’s Law
- The Mechanical and Electrical Dynamics of Gel Electrophoresis – Intro and Sample Mobility
- The Electrophoresis Matrix
- The Agarose Matrix
- Radioactive Emissions and the Use of Isotopes in Research
- Multiphasic Buffer Systems
- Horizontal and Vertical Gel Systems – Vertical Tube Gels
- Horizontal and Vertical Gel Systems – The Vertical Slab Gel System
- Horizontal and Vertical Gel Systems – The Horizontal Gel System
- Homogeneous Buffer Systems
- Faint bands, low background
- Faint Bands, High Background
- Ethidium Bromide Staining
- Electrophoresis Buffers-Choosing the Right Buffer
- Electrophoresis Buffers–The Henderson-Hasselbalch Equation
- Coomassie Blue Stain- Troubleshooting
- Buffer Additives-Surfactants
- Buffer Additives-Reducing Agents
- Buffer Additives-Hydrogen Bonding Agents
- Biological Macromolecules: Nucleic Acids
- Biological Macromolecules – Proteins
