Post Electrophoretic Analysis Articles
Multiphasic Buffer Systems
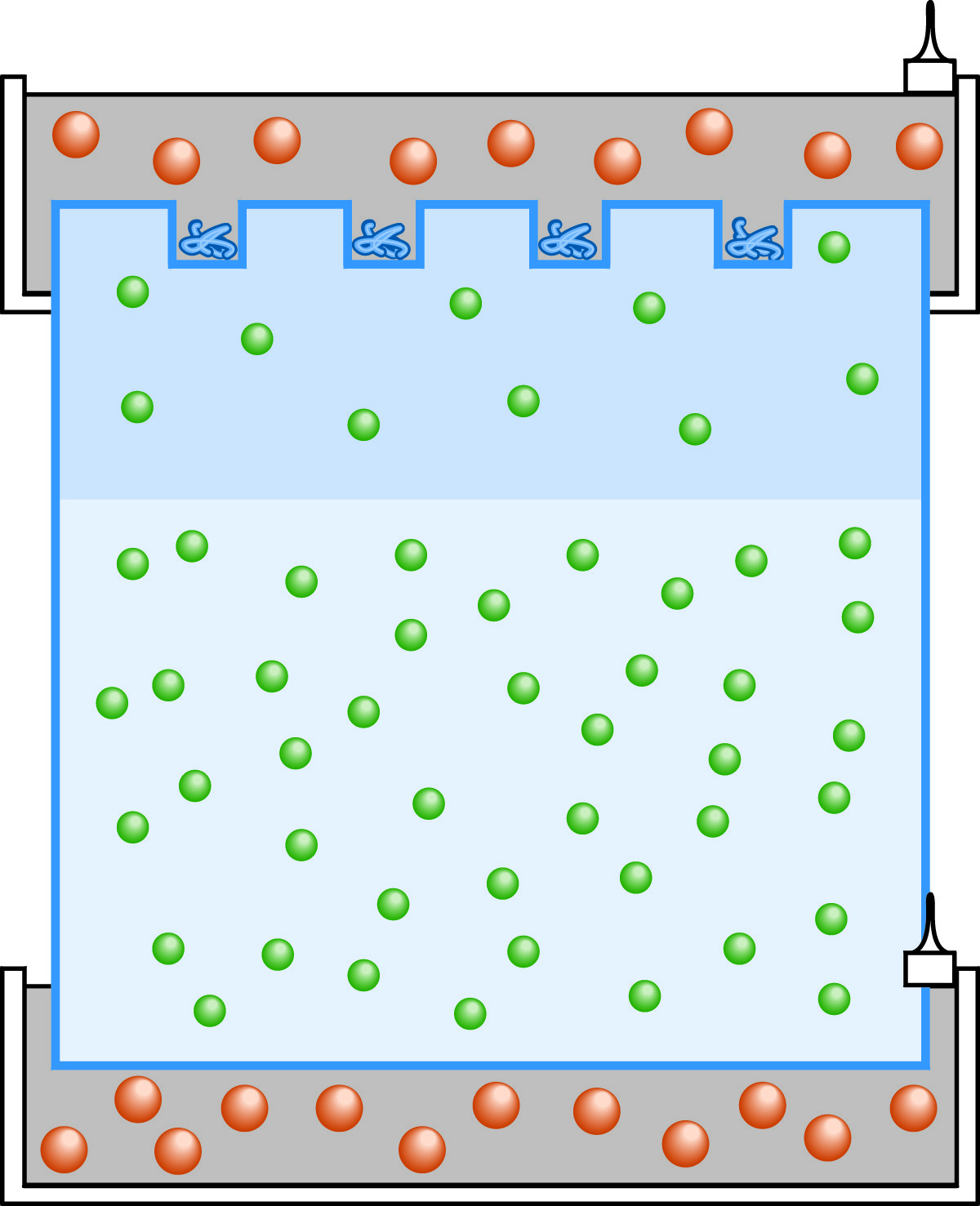
At the start of multiphasic SDS-PAGE protein electrophoresis, the anions are chloride (green) in the stacking and resolving gels and glycine (orange) in the tanks.
Employing gel and buffer discontinuities to produce sharp separation among sample components, multiphasic electrophoresis design can improve the resolution of electrophoresis (especially protein electrophoresis). The system employs a separating gel in which the sample is fractionated, above which has been added a low percentage stacking gel. In the stacking gel, sample components are stacked into very thin, sharp zones prior to the final separation. To illustrate the principles of multiphasic systems, we shall examine the most prominent application of this type, the SDS-PAGE electrophoresis of proteins. Multiphasic SDS-PAGE protein electrophoresis is often referred to as the Laemmli system after the researcher who perfected the design. At the beginning of the experiment, the buffer compositions are different in the stacking gel, the separation gel, and the tank. The stacking gel contains a Tris-HCl buffer at pH 6.8. The separation gel contains a higher concentration Tris-HCl buffer at pH 8.8. The tanks contain Tris-glycine at pH 8.8.
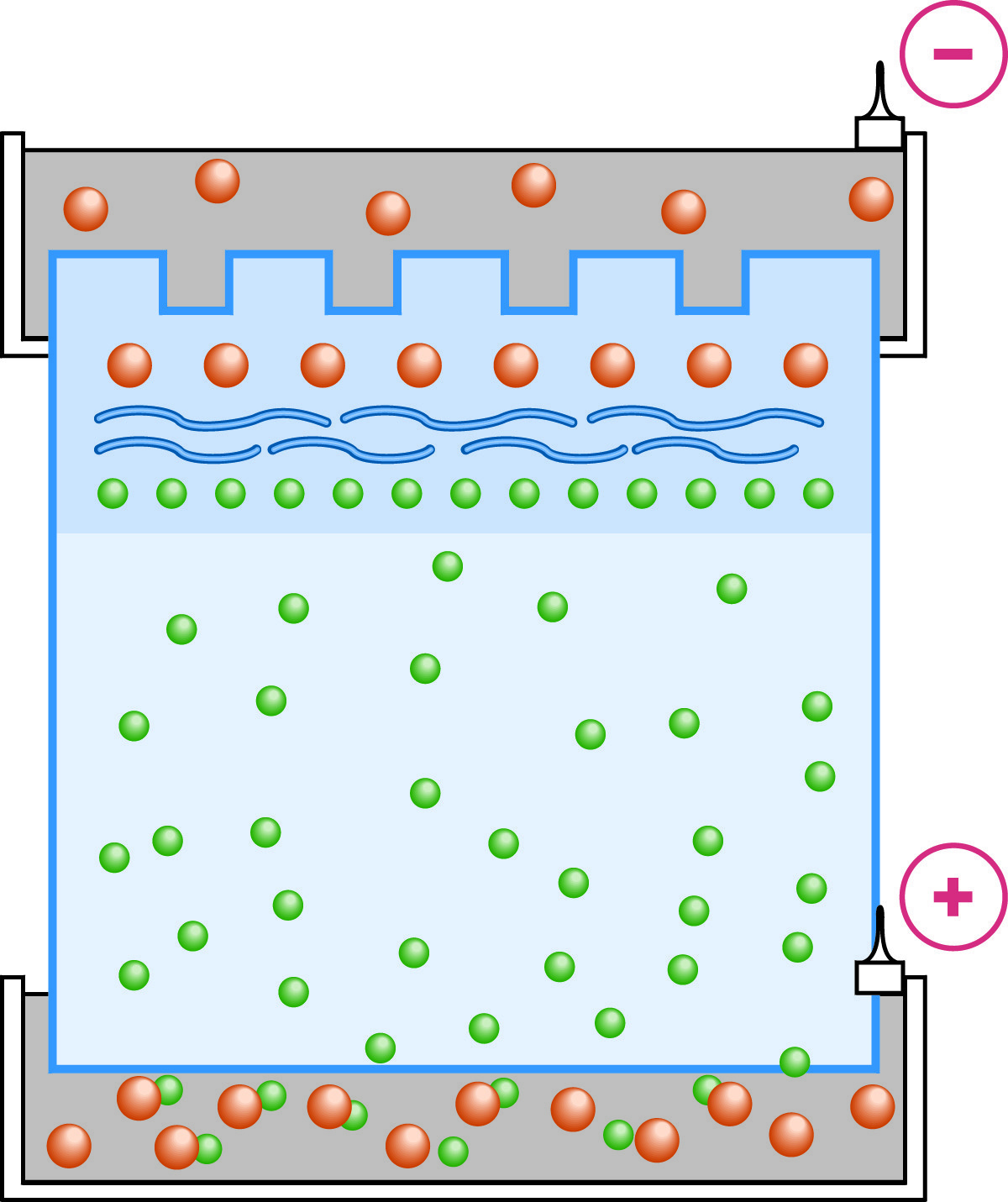
With the establishment of the Kohlrausch boundary in the stacking gel, the proteins are stacked into a thin layer between the leading chloride ions and the trailing glycine molecules.
As electrophoresis begins both Cl- and glycinate ions begin to migrate through the stacking gel. Because the pH is several points lower in the stacking gel than the pKa2of glycine, the vast majority of glycine molecules are zwitterionic at any moment and their mobility is very low. Because the mobility of the chloride ions is greater than the glycine, the chloride ions (leading ions) begin to migrate away from the glycine (trailing ions). The chloride ions don't move far before they leave behind an area of unbalanced positive counter ions. A steep voltage gradient develops, the Kohlrausch discontinuity, which pulls the glycine along so that the chloride and glycine ions become successive fronts moving at the same speed. The ion fronts sweep through the sample molecules. Being intermediate in their mobility between chloride and glycine, the sample molecules are carried along becoming "stacked" into very thin, distinct layers in order of electrophoretic mobility.
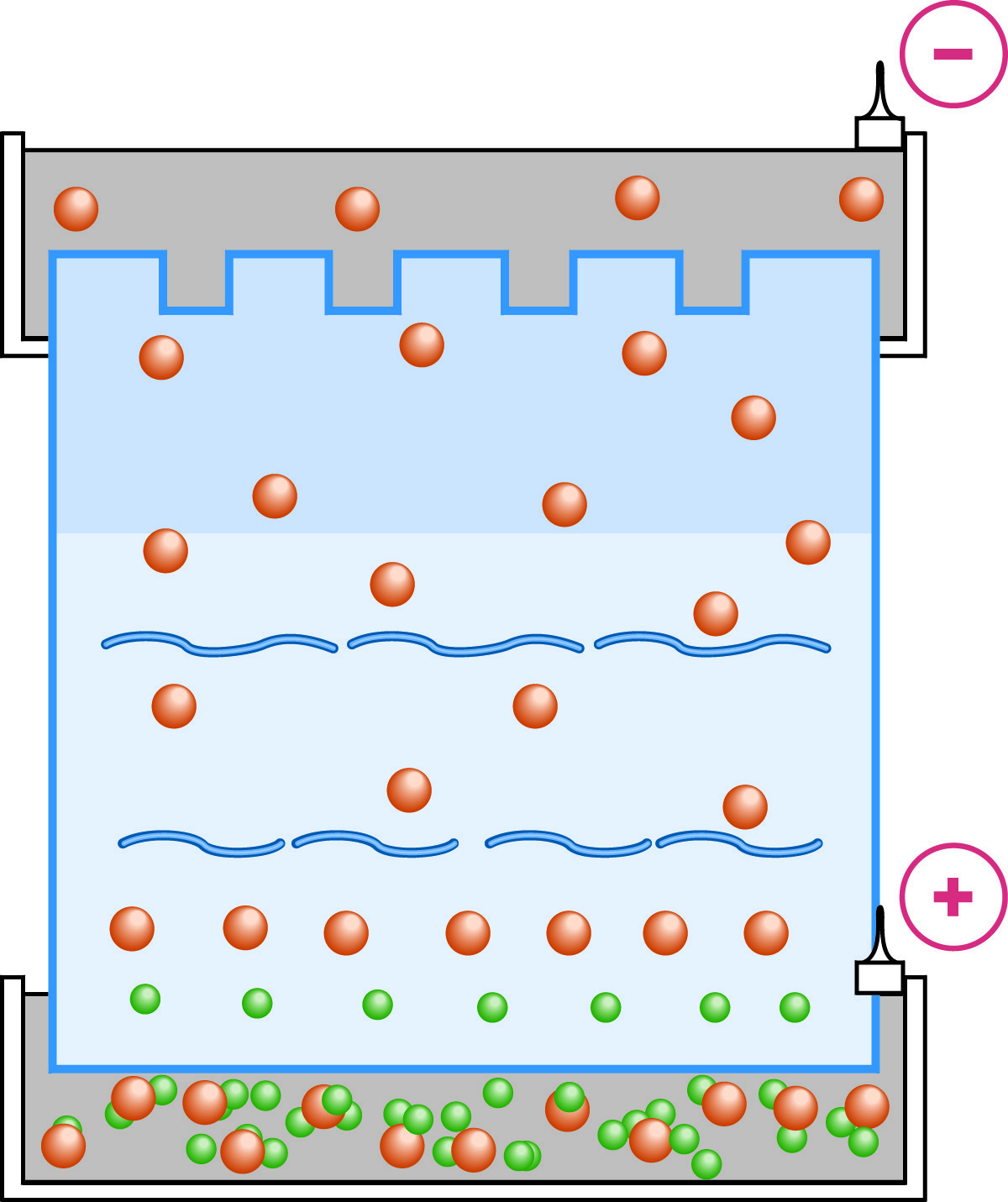
Electrophoresis in the resolving gel is indistinguishable from ordinary homogeneous buffer systems.
When the interface between the stacking and separation gels is reached by the moving boundary region, the pH changes abruptly (as well as the pore size). At the higher pH a much higher percentage of glycine will be in the ionized state, and its mobility will be increased commensurately. The mobility of sample molecules is decreased by the sieving of the new, higher percentage matrix. The glycine accelerates past the stacked layers of sample molecules and the process of unstacking in the separating gel begins. From this point on, the electrophoretic separation occurs under conditions of constant pH and voltage that are indistinguishable from homogeneous PAGE.
NEXT TOPIC: Isotachophoresis
- The Polyacrylamide Matrix-Buffer Strength
- The Polyacrylamide Matrix
- The Mechanical and Electrical Dynamics of Gel Electrophoresis — Electrophoresis System Dynamics
- The Mechanical and Electrical Dynamics of Gel Electrophoresis – Ohm’s Law
- The Mechanical and Electrical Dynamics of Gel Electrophoresis – Intro and Sample Mobility
- The Electrophoresis Matrix
- The Agarose Matrix
- Radioactive Emissions and the Use of Isotopes in Research
- Multiphasic Buffer Systems
- Horizontal and Vertical Gel Systems – Vertical Tube Gels
- Horizontal and Vertical Gel Systems – The Vertical Slab Gel System
- Horizontal and Vertical Gel Systems – The Horizontal Gel System
- Homogeneous Buffer Systems
- Faint bands, low background
- Faint Bands, High Background
- Ethidium Bromide Staining
- Electrophoresis Buffers-Choosing the Right Buffer
- Electrophoresis Buffers–The Henderson-Hasselbalch Equation
- Coomassie Blue Stain- Troubleshooting
- Buffer Additives-Surfactants
- Buffer Additives-Reducing Agents
- Buffer Additives-Hydrogen Bonding Agents
- Biological Macromolecules: Nucleic Acids
- Biological Macromolecules – Proteins
