Advanced Histological Techniques
Immunohistochemistry
Immunohistochemistry is the application of antibody/antigen interactions to provide information about biological systems. The body's response to the introduction of a foreign agent, known as the immune response, results in the production of antibodies which bind the offending material. Antibodies bind tightly and specifically to an "epitope" (one specific structure) on an "antigen" (foreign molecule or structure).
An antigen can be defined as "anything that can be bound by an antibody." This can be an enormous range of substances from simple chemicals, sugars, and small peptides to complex protein complexes such as a virus capsid.
Not all antigens directly elicit an antibody response. Some require a carrier to be effective. These generally smaller antigens are called haptens.
The structure of an antibody resembles a 'Y'. The stem of the 'Y' is the "constant region," which defines the animal and class of the antibody. Antibody classes are designated by a letter, and prefixed with Ig (for immunoglobulin), thus IgG, IgM and IgE are all classes of antibodies, and all human IgG molecules have the same constant region. The arms of the 'Y' contain the variable regions of the antibody, where the antigen binding sites are located. Each arm has a binding site, so each antibody can bind two antigens.
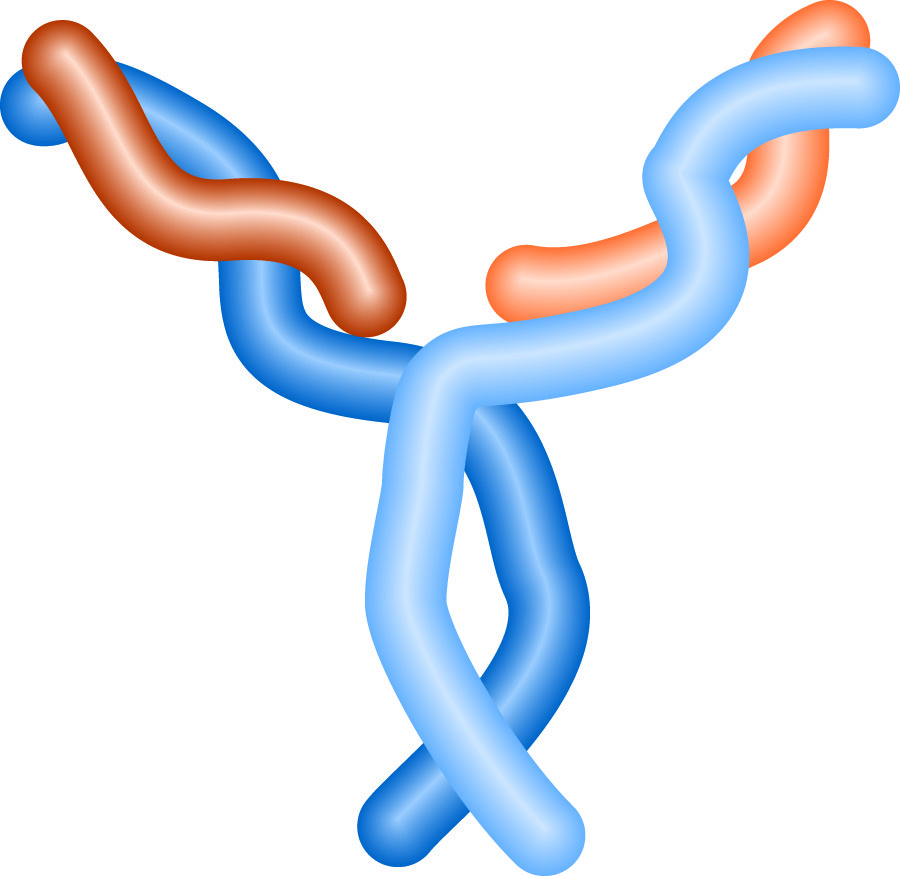
Antibody Structure.
Antibodies can be generated by injecting animals with antigens, and then collecting serum after the immune response has taken place. If the antibodies are labeled with an easily detectable molecule (a fluorescent dye, an enzyme, etc.), they become powerful detection reagents for the antigen. This system has been exploited to generate exceptionally specific and sensitive "stains" which are used in histology as well as other disciplines.
Immunohistochemistry/cytology is the use of antibodies in light microscopy and EM. The basic process depends upon selecting an antibody sufficiently specific to bind an antigen in situ. The antibody/antigen conjugate is then identified using a variety of signal generating molecules triggered either by the antibody/antigen interaction or by secondary processes. The signal generators can be precipitating dyes, fluorescent molecules or electron dense (ultrastructural tag) materials for electron microscopy (EM).
The first report of an immunohistochemistry technique was made in 1942 when Coons et al detected pneumococcal antigen using a fluorescently tagged antibody. The immunohistochemical techniques for EM were developed by Singer using ferritin (1959). This was quickly followed by the first use of an enzyme, horseradish peroxidase (still widely used) in 1966 by Graham et al. Since then, a variety of enzyme and heavy metal techniques have been developed, the most important of which are Colloidal Gold for EM (Faulk 1971), Immunoperoxidase assay (Nakane, 1966), peroxidase/anti-peroxidase PAP technique (Sternberger 1970) and the Avidin-Biotin Complex (ABC) technique (Hsu, 1981).
Immunohistochemistry is generally carried out in sectioned tissue, which allows the antibodies free access to the interior of the cells. Immunohistochemistry can also be carried out on cells either in free solution or bound to membranes, or on monolayers of cultured cells. Intracellular Immunohistochemistry requires that the antibody to the target antigen be able to penetrate the cell membrane and whatever cell wall may be present before it can attach to the antigen. This requires a number of steps not required for sectioned tissue. Primarily the cell membrane must be made permeable to the antibody, though at the same time the integrity of the cell contents and structures must be maintained. This is normally achieved through the use of a specialized buffer containing a detergent. Saponin is frequently used for this purpose.
NEXT TOPIC: Antibody Binding
