Electrophoresis
ProtoBlue Safe
Price range: $71.00 through $305.00
Get a free sample of ProtoBlue Safe! Call us at (800) 526-3867 for details!
- Fast, Easy to Use Protocol
- Ecofriendly; Nonhazardous disposal
- Costs Less than Regular Coomassie
Description
Get a free sample of ProtoBlue Safe! Call us at (800) 526-3867 for details!
- Fast, Easy to Use Protocol
- Ecofriendly; Nonhazardous disposal
- Costs Less than Regular Coomassie
The Best Performing Colloidal Stain – Detects as little as 5ng protein
Compared to similar stains, the more finely controlled colloidal structure of ProtoBlue Safe improves both the sensitivity and the universality of staining. ProtoBlue Safe is less prone to high background caused by trace residual SDS in the gel.
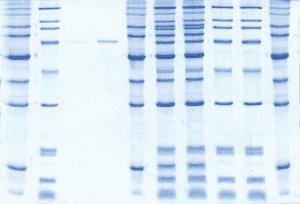
Costs Less than Regular Coomassie
Laboratories typically spend twice as much per gel in the methanol and acetic acid for staining and destaining with regular Coomassie than for ProtoBlue Safe (including ethanol cost). In addition, ProtoBlue Safe is much faster, more sensitive, and you can pour the used stain solution down the drain. Protein visualization is faster, safer, more sensitive and less expensive with ProtoBlue Safe.
Nonhazardous Disposal
Used stain solution is not a hazardous waste (as defined by United States Title 40 Code of Federal Regulations (40 CFR 261.24(a)).
Fast Protocol – No Methanol or Acetic Acid
Your sample can be detected with these three quick, easy steps:
- Wash gel three times for five minutes with deionized water on an orbital shaker.
(Note: This step is optional. A longer incubation in stain solution and longer post-stain washing can substitute for this wash step.) - Add one part ethanol to nine parts staining solution while stirring. Adding ethanol greatly improves sensitivity, reproductivity and shelf life compared to solutions that add ethanol at point of manufacture.
- Add enough staining solution to completely cover the gel. Bands containing more than 1 μg will be detected within 15 minutes.
(Note: For sensitivity up to 5 ng, incubate the gel in stain for at least five hours.)
Additional information
| Weight | 0.1 lbs |
|---|---|
| Dimensions | 8 × 8 × 16 in |
Protocol
Prepare Working Solution
- Gently invert the bottle several times to resuspend colloidal dye particles that settle out on standing.
- Add 1 part ethanol to 9 parts staining solution while stirring. (Standard denatured ethanol is fine). A 20ml to 50ml volume of working solution is typically prepared, depending on the shape and size of the staining container. To preserve solution, we recommend a plastic staining container just big enough to hold the gel.
Procedures for Gel Staining
- Prepare ProtoBlue Safe Working Solution (above).
- Wash the gel 3 times with 5 minutes each with deionized water on an orbital shaker. Decant wash solution.
- After the last wash, add enough ProtoBlue Safe Working Solution to completely cover the gel.
- Bands containing more than 1μg of protein will be detected within 15 minutes. For full sensitivity incubate the gel in the stain for at least 4-5 hours. Longer incubations in the stain will not adversely affect the gel or the staining sensitivity.
- Remove the stain and wash the gel in deionized water. Incubating the gel in water increases the sensitivity of detection by reducing the background to crystal clear. The gel is stable in water for up to a week without loss of sensitivity. There is no need to store the gel in a salt solution.
Safety Overview
Safety Summary (see SDS for complete information before using product):
Appearance and Odor
Blue solution.
EMERGENCY OVERVIEW – IMMEDIATE HAZARD
MAY CAUSE IRRITATION TO SKIN AND EYES. DUST LEFT AFTER SOLVENT EVAPORATION MAY CAUSE IRRITATION TO THE RESPIRATORY TRACT.
- UV Shadowing
- Using PAGE to Determine Nucleic Acid Molecular Weight
- Uneven Staining
- The Polyacrylamide Matrix-Buffer Strength
- The Polyacrylamide Matrix
- The Mechanical and Electrical Dynamics of Gel Electrophoresis — Electrophoresis System Dynamics
- The Mechanical and Electrical Dynamics of Gel Electrophoresis – Ohm’s Law
- The Mechanical and Electrical Dynamics of Gel Electrophoresis – Intro and Sample Mobility
- The Electrophoresis Matrix
- The Agarose Matrix
- Staining Proteins Immobilized on Membranes
- Staining Protein Gels with Coomassie Blue
- SSCP Analysis
- Southern Blotting
- Smeared Bands
- Silver Staining Protein Gels
- Silver Staining DNA Gels
- Sanger Sequencing
- Sample Preparation for SDS-PAGE
- Sample Preparation for Native Protein Electrophoresis
- Sample Preparation for Native PAGE of DNA
- Sample Prep for Denaturing PAGE of DNA
- S1 Mapping
- Run Conditions in Denaturing PAGE
- RNA Mapping
- RNA Electrophoresis
- Ribonuclease Protection
- Restriction Digest Mapping
- Radioactive Emissions and the Use of Isotopes in Research
- Protein Fixation on Gels
- Primer Extension
- Preparing Denaturing DNA & RNA Gels
- Preparation of Denaturing Agarose Gels
- Preparation of Agarose Gels
- Pouring Sequencing Gels
- Post-Electrophoretic Visualization with Nuclistain
- PFGE and FIGE
- Peptide Mapping
- PCR Analysis: Yield and Kinetics
- PCR Analysis: An Examination
- Overview of Western Blotting
- Northern Blotting
- Native Protein Electrophoresis
- Native PAGE of DNA
- Multiphasic Buffer Systems
- Mobility Shift Assay
- Methylation & Uracil Interference Assays
- Method for Western Blotting
- Mechanism of Immunostaining
- Mechanism of Immunostaining
- Measuring Molecular Weight with SDS-PAGE
- Maxam & Gilbert Sequencing
- Manual Sequencing
- Isotachophoresis
- Isoelectric Focusing
- In Gel Enzyme Reactions
- Immunostaining with Alkaline Phosphatase
- Immuno-Electrophoresis / Immuno-Diffusion
- Horizontal and Vertical Gel Systems – Vertical Tube Gels
- Horizontal and Vertical Gel Systems – The Vertical Slab Gel System
- Horizontal and Vertical Gel Systems – The Horizontal Gel System
- Homogeneous Buffer Systems
- Heteroduplex Analysis
- Guide Strip Technique
- Gel Preparation for Native Protein Electrophoresis
- Gel Preparation for Native PAGE of DNA
- Gel Electrophoresis of RNA & Post Electrophoretic Analysis
- Gel Electrophoresis of PCR Products
- Faint bands, low background
- Faint Bands, High Background
- Ethidium Bromide Staining
- Enzyme Linked Immunosorbent Assay (ELISA)
- Electrophoresis Buffers-Choosing the Right Buffer
- Electrophoresis Buffers–The Henderson-Hasselbalch Equation
- DNase I Footprinting
- DNA/RNA Purification from PAGE Gels
- DNA/RNA Purification from Agarose Gels – Electroelution
- Differential Display
- Denaturing Protein Electrophoresis: SDS-PAGE
- Denaturing Polyacrylamide Gel Electrophoresis of DNA & RNA
- Coomassie Blue Stain- Troubleshooting
- Conformational Analysis
- Casting Gradient Gels
- Buffer Additives-Surfactants
- Buffer Additives-Reducing Agents
- Buffer Additives-Hydrogen Bonding Agents
- Blotches on Gel
- Biological Macromolecules: Nucleic Acids
- Biological Macromolecules – Proteins
- Autoradiography
- Autoradiographic Enhancement with Autofluor
- Automated Sequencers
- Analysis of DNA/Protein Interactions
- An Overview of Northern and Southern Blotting
- Alkaline Blotting
- Agarose Gel Electrophoresis of DNA and RNA – Uses and Variations
- Agarose Gel Electrophoresis of DNA and RNA – An Introduction
- Activity Stains